Introduction
Use of the two commercially available anti CD19 chimeric antigen receptor T-cell (CAR T-cell) therapies in patients with relapsed and refractory (R/R) large B-cell lymphoma (LBCL) is associated with significant inpatient and outpatient resource utilization, which has not been systematically compared. We characterized institutional resources used around Tisagenlecleucel (tisa-cel) and Axicabtagene ciloleucel (axi-cel) infusion and identified factors that led to longer hospitalization during the first 100 days of therapy.
Methods
We reviewed medical records of consecutive adult patients with R/R LBCL treated with tisa-cel or axi-cel at our center from May 2018 to May 2020. Resource utilization data included inpatient hospital days, outpatient clinic visits, radiological studies, cardiac/neurologic studies, invasive procedures and pharmaceutical resources. "Days alive and out-of-hospital at Day 100" (DAOH100), were defined as the number of living days that a patient spent out-of-hospital, which served as a surrogate for institutional resource utilization, as it accounted for duration of index hospitalization, re-admissions and days lost due to premature death, within first 100 days of therapy. Data were collected from the day of leukapheresis through Day 100 post CAR T-cell infusion (day 0), and compared between tisa-cel and axi-cel recipients.
Results
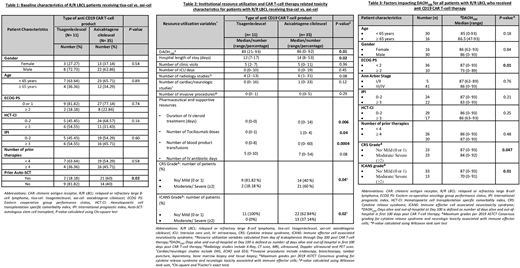
Of 46 patients with R/R LBCL, axi-cel was used in 35 (76%) and tisa-cel in 11 (24%) patients. 30 (65%) patients were male, 44 (96%) were Caucasian, with median age of 61 (range, 25-77) years and 3 (range, 2-6) prior lines of therapy. Forty- one (89%) patients had stage III/IV disease, 24 (52%) had IPI 0-2, 36 (78%) had ECOG PS 0 or 1 and 29 (63%) had HCT-CI <3. Baseline characteristics were comparable among tisa-cel and axi-cel recipients, except axi-cel group having more patients with prior autologous transplant (Table 1).
Median duration of hospitalization was 14 (range, 7-53) days, with 13 (28%) patients requiring ICU admission for a median of 3 days (range, 1-19). The median number of outpatient clinic visits, radiology studies, cardiac/neurologic evaluations and invasive diagnostic procedures through Day 100 were 5 (range, 0-11), 6 (range, 1- 31), 1 (range, 0-33) and 0 (range, 0-5), respectively. Patients required a median of 1 (range, 0-4) dose(s) of tocilizumab, 0 (range, 0-14) days of IV steroids, 7 (range, 0- 54) days of IV antibiotics and 7 (range, 0-60) blood product transfusions. When compared to patients receiving axi-cel, the tisa-cel group had significantly shorter hospital stay (P= 0.02), shorter IV steroid days (P= 0.006), fewer tocilizumab doses (P=0.04) and fewer blood product transfusions (P=0.0004) (Table 2).
Cytokine release syndrome (CRS) and immune effector cell mediated neurotoxicity syndrome (ICANS) were seen in 35 (76%) and 23 (50%) patients, respectively. In comparison to axi-cel, significantly fewer patients with tisa-cel had moderate to severe (≥ Grade 2) CRS (18.18 % vs. 60%; P= 0.04), and moderate to severe (≥ Grade 2) ICANS (0% vs. 37.14%; P= 0.02) (Table 2). By Day 100, 15 (32.6%) patients had died and 4 (10.8%) had disease progression. The median DAOH100 were 86 (range, 0-93) days. Patients receiving tisa-cel experienced longer median DAOH100 compared to those receiving axi-cel (89 vs. 86; P= 0.01) (Table 2). DAOH100 were higher for patients with favorable ECOG PS (0 or 1) vs. unfavorable (≥ 2) (median of 87 vs 73 days, P=0.01). Patients with no/mild CRS, and those with no/mild ICANS, had longer DAOH100 vs. those with ≥ Grade 2 CRS and ≥ Grade 2 ICANS (median of 87 vs. 84 days; P= 0.047 & median of 87 vs. 70 days; P= 0.01), respectively. DAOH100 did not differ with respect to age, gender, stage, HCT-CI, IPI or number of prior therapies (Table 3).
Conclusion
Through the first 100 days of CAR T-cell therapy, R/R LBCL patients receiving tisa-cel had shorter hospital length of stay, more days alive and out-of-hospital, received fewer tocilizumab doses, steroid days and blood product transfusions, compared to those treated with axi-cel. Patients receiving tisa-cel also experienced lesser incidence of moderate/severe CRS and ICANS. Favorable ECOG PS and absence of moderate/severe CRS or ICANS significantly increased DAOH100. In light of these differences in toxicity and resource utilization, a future study is needed to compare efficacy of axi-cel vs. tisa-cel in larger patient cohorts.
Hill:Genentech: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Hamilton:Syndax Pharmaceuticals: Consultancy, Honoraria. Khouri:Sanofi Genzyme: Other: Advisory Board. Jagadeesh:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Debiopharm Group: Research Funding; Verastem: Membership on an entity's Board of Directors or advisory committees; Regeneron: Research Funding; MEI Pharma: Research Funding. Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau. Gerds:CTI Biopharma: Consultancy, Research Funding; Roche/Genentech: Research Funding; Incyte Corporation: Consultancy, Research Funding; AstraZeneca/MedImmune: Consultancy; Apexx Oncology: Consultancy; Celgene: Consultancy, Research Funding; Gilead Sciences: Research Funding; Imago Biosciences: Research Funding; Sierra Oncology: Research Funding; Pfizer: Research Funding. Majhail:Mallinckrodt: Honoraria; Anthem, Inc.: Consultancy; Nkarta Therapeutics: Honoraria; Incyte: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal